Biotech Research & Development Support Services

Independent Bench/Field Trials Testing
As our global society continues to increase the scrutiny in R&D systems, third party independent bench testing has become a necessity in demonstrating construct validity and test method reliability for new products and processes. Products filling the corporate R&D pipeline that are competing for patent and registration can often tie up corporate R&D laboratories even as the available corporate facilities and staff continue to shrink.
We offer coordination of independent bench testing for a wide cross-section of industry, including both chemical and physical testing of products. We partner with only the following accredited laboratories (ISO 17025 and state-accredited), while providing the necessary project management and data validation to ensure your product has the strongest, legally defensible data:
We partner with only the following research-focused custom manufacturers and blenders for the following types of ingredients and products:
For natural health, human kinetic, and nutraceutical (medicinal foods) studies we design and conduct research studies for either in vitro or in vivo lab applications, volunteer testing populations, and peer review of testing methodology and data analysis. As such, your biotech product has the vehicle necessary to isolate general or target test populations with our terminally degreed research pharmacists (PharmD).
Custom Environmental Solutions, LLC is our strategic partner source provider for all biotech product spin-ups, as they contribute a collaboration team with some of the most advanced and innovative abilities for critical chemical and biochemical ingredients (including a patented molecule for various heavy industry and human personal care product applications) available on the globe. Collectively, we are able to effectively help you to produce customized ingredient options for almost any innovative R&D product idea, leading to bench trials, field testing, and ultimately full-scale manufacturing for market entry.
We offer all R&D independent bench testing services with only Ph.D. level project management and data validation.
*Please also see our Engineering & Field page.
As our global society continues to increase the scrutiny in R&D systems, third party independent bench testing has become a necessity in demonstrating construct validity and test method reliability for new products and processes. Products filling the corporate R&D pipeline that are competing for patent and registration can often tie up corporate R&D laboratories even as the available corporate facilities and staff continue to shrink.
We offer coordination of independent bench testing for a wide cross-section of industry, including both chemical and physical testing of products. We partner with only the following accredited laboratories (ISO 17025 and state-accredited), while providing the necessary project management and data validation to ensure your product has the strongest, legally defensible data:
- Mr. DNA Lab (quantitative genetic testing and foot-printing)
- Twin Arbor Laboratories (nutraceutical, personal care products, and food testing)
- B.A.T. Laboratories (broad topic quantitative R&D bench studies/trials, and testing)
We partner with only the following research-focused custom manufacturers and blenders for the following types of ingredients and products:
- 3 Tier Technologies (agricultural and environmental biopolymer R&D)
- Custom Environmental Solutions, LLC (chemical/biochemical ingredients R&D)
- BioChem Systems (heavy industrial chemical ingredients R&D)
For natural health, human kinetic, and nutraceutical (medicinal foods) studies we design and conduct research studies for either in vitro or in vivo lab applications, volunteer testing populations, and peer review of testing methodology and data analysis. As such, your biotech product has the vehicle necessary to isolate general or target test populations with our terminally degreed research pharmacists (PharmD).
Custom Environmental Solutions, LLC is our strategic partner source provider for all biotech product spin-ups, as they contribute a collaboration team with some of the most advanced and innovative abilities for critical chemical and biochemical ingredients (including a patented molecule for various heavy industry and human personal care product applications) available on the globe. Collectively, we are able to effectively help you to produce customized ingredient options for almost any innovative R&D product idea, leading to bench trials, field testing, and ultimately full-scale manufacturing for market entry.
We offer all R&D independent bench testing services with only Ph.D. level project management and data validation.
*Please also see our Engineering & Field page.
Exhaustive Stacks Research
The introduction of the internet created an entirely new paradox in peer-reviewed research; global informatics. What formerly constituted fundamental research of existing academic literature through paper journals and books has now been outplaced with electronic stacks. Global academic search engines and Boolean operating systems have effectively replaced largely domestic peer reviewed academic literature. The result is the need to access global, published research, and a consequentially increased difficulty conducting exhaustive literature review of academic literature.
We offer the research expertise with electronic database research to conduct exhaustive literature reviews of peer reviewed academic literature, complete with annotated bibliographies, summaries, and any necessary reference material within our report. Let us take the preliminary research step for you while you and your staff spend your critical time further developing your innovative new technology or product.
*Please also see our Engineering & Field page.
Technical Sheet Reviews
Product sheets, technical data sheets, safety data sheets (SDS), Standard Operating Procedures (SOP), white papers, technical presentations, and other literature necessary to accurately and legally describe and subsequently launch a new product or technology is the final critical step in any R&D system. Without the assurance that what you have described accurately depicts your product's intended use, tolerances, reflective limitations, and benefits the product launch is potentially compromised. You recognize the need for third party validation of technical sheet review. We are researchers like you. We understand this need.
We offer an advanced level of peer review of your product's technical data sheets. After a thorough review of your product and related performance data we generate a summary report of perceived grammatical, mathematical, and scientific misprints with clear reference to your own submitted performance data and product summary.
*Please also see our Engineering & Field page.
Process Investigation
Contemporary society relies heavily on internal processes that drive innovative R&D. These processes are nothing more than a series of complex hybrid systems comprised of people systems, electronic systems, chemical systems, and business systems that must work in tandem to produce the intended result. Often these systems become so complex that internal staff lack the perspective to objectively evaluate these systems as a complete process. Outsourcing this external perspective has a demonstrated history of allowing internal staff to accurately and quickly identify root cause, recognize opportunities to reduce complexity, and subsequently increase research or production velocity and efficacy.
As an out-sourced consultant we offer the blended academic and experiential perspective of the combined disciplines of engineering, natural science, environmental and safety, and business. We understand people systems as well as mechanistic systems. We understand loss prevention as well as productivity and statistical process control. We will enhance your own efforts with our skill sets, affording you the opportunity to maintain control of your investigation while leveraging your strengths with our own.
*Please also see our Engineering & Field page.
Design of Experiments
While mature organizations typically staff experienced researchers many start-up organizations are centered around an innovative new idea/product and the innovator behind that technology. As such, helping to differentiate and document their new product with statistical certainty may be a challenge for some. Understanding what testing methodology to apply as an appropriate metric of performance, with the proper level of acceptable uncertainty, is doctoral level research.
We offer to sit down with you to learn about your product, understand what question(s) need to be answered, and develop a quantitatively designed experiment to evaluate and ultimately validate your product's unique aspects that differentiate it from competing or existing products or technologies. Finally, we present you with the raw data and analysis for you to either develop or allow us to develop into patent applications, marketing materials, and technical data sheets. Through the design of experiments we facilitate your taking a product or idea to market without the loss of valuable time and resources from your own staff.
We offer all R&D design of experiment services with only Ph.D. level project management and data validation.
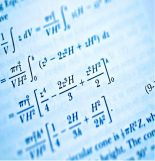
Quantitative/Qualitative Statistical Analysis
Interpretation of collected data is arguably the most difficult step in the research process. Qualitative statistical analysis and quantitative data reduction often requires sophisticated statistical methods in order to account for perceived anomalies, unequal population variances, sub-sampling data variance, canonical correlations among variables, and skewed data sets.
We offer your research program doctoral level statistical analysis with proven commercial statistical software. Further, we specialize in statistical frustrations, such as limited population and sample sizes, unequal population variances, non-parametric data sets, and highly abnormally distributed data. An additional specialization is meta-analysis for a wide range of life science research areas/disciplines. Let us perform your statistical analysis on your most mundane or troublesome data sets.
*Please also see our Engineering & Field page.
Performance Gap Analysis
Performance testing of products, processes, or people often evade those scientists, managers, and executives closest to the system. Many times, performance gaps are most efficiently determined by a third party, given the absence of bias and the presence of comparative experiential observation.
We offer your system (manufacturing, scientific, personnel, or business) cutting edge performance gap analysis based on contemporary academic models. Let us evaluate your system and identify performance improvement opportunities, while working closely with you to identify your own best-in-class system aspects for replication within your own organization.
Data Validation
Critical data generated by your R&D process needs to demonstrate validity and reliability. Peer reviewing your data is a necessary step in your process. Third party review of your data requires the most qualified analysts.
We offer complete data validation, to include external audit criteria (i.e., U.S. EPA, USDA, etc.). Focus on other work while we do the critical last step of ensuring your data is plausible, sound, and legally defensible.
We are pleased to initiate and execute client Confidentiality Agreements prior to project assessment.
Interpretation of collected data is arguably the most difficult step in the research process. Qualitative statistical analysis and quantitative data reduction often requires sophisticated statistical methods in order to account for perceived anomalies, unequal population variances, sub-sampling data variance, canonical correlations among variables, and skewed data sets.
We offer your research program doctoral level statistical analysis with proven commercial statistical software. Further, we specialize in statistical frustrations, such as limited population and sample sizes, unequal population variances, non-parametric data sets, and highly abnormally distributed data. An additional specialization is meta-analysis for a wide range of life science research areas/disciplines. Let us perform your statistical analysis on your most mundane or troublesome data sets.
*Please also see our Engineering & Field page.
Performance Gap Analysis
Performance testing of products, processes, or people often evade those scientists, managers, and executives closest to the system. Many times, performance gaps are most efficiently determined by a third party, given the absence of bias and the presence of comparative experiential observation.
We offer your system (manufacturing, scientific, personnel, or business) cutting edge performance gap analysis based on contemporary academic models. Let us evaluate your system and identify performance improvement opportunities, while working closely with you to identify your own best-in-class system aspects for replication within your own organization.
Data Validation
Critical data generated by your R&D process needs to demonstrate validity and reliability. Peer reviewing your data is a necessary step in your process. Third party review of your data requires the most qualified analysts.
We offer complete data validation, to include external audit criteria (i.e., U.S. EPA, USDA, etc.). Focus on other work while we do the critical last step of ensuring your data is plausible, sound, and legally defensible.
We are pleased to initiate and execute client Confidentiality Agreements prior to project assessment.
CURRENT RESEARCH DEVELOPED BY ADVANCED BIOGENETICS RESEARCH STAFF
*Please contact us to request a full copy of the paper
*Please contact us to request a full copy of the paper
- Baumgardner, P. (LONGITUDINAL STUDY; IN PROGRESS starting January 2023 to current). Impact of red yeast rice stacked with CoQ10, milk thistle, and alpha lipoic acid on cholesterol balance as a safe and natural statin alternative in middle-aged athletic males.
- Reasoner, D., Rogers, J., Baumgardner, P., Welbaum, J., & Flynn, N. (2017). Analysis of panhandle playa lake water for contamination residuals. American Chemical Society: Southwest Regional Meeting. Lubbock, TX
- Flynn, N., Reasoner, D., Read, J., & Baumgardner, P. (2018). Home water analysis kits: Comparisons to laboratory methods and implications for home brewing. Zymurgy. 41(2), 46-53
- Baumgardner, P. (LONGITUDINAL STUDY; IN PROGRESS starting May 2012 to current). General human health, wellness, and sports fitness performance outcomes utilizing natural dietary supplements, foods, cannabinoids, and exercise strategies.
- Flynn, N., Reasoner, D., Read, J., & Baumgardner, P. (2016). Comparison of a brewer's water analysis kit to standardized methods and implications for brewing. American Chemical Society: National Meeting. San Diego, CA
- Flynn, N.E. and Baumgardner, P. (2015). Effect of glass color on IBU perception and IBU measured value on beer exposed to UV light. American Chemical Society: Southwest Regional Meeting. Memphis, TN
- Catherine, C. and Flynn, N.E. (2015). Using an on-site liquid nitrogen generator for NMR cryogen supply and other departmental needs. Journal Chemistry Education. 92, 589-592
- Whitener, W. (2015). Validity in research.
- Whitener, W. (2015). Ethical implications of research.
- Pruser, K. and Flynn, N.E. (2011). Acrylamide in health and disease. Frontiers in Bioscience. S1, 41-51.
- Flynn N.E. (2010) Design your own disease: Teaching students to apply metabolic pathways. Journal of Chemical Education. doi: 10.1021/ed100279z.
- Flynn N.E., Patyrak M., Seely J., Wu G. (2010). Glycine oxidation and conversion into amino acids in Saccharomyces cerevisiae and Candida albicans. Amino Acids. doi: 10.1007/s00726-010-0477-7.
- Flynn N.E., Bird J.G., Guthrie A.S. (2009). Glucocorticoid regulation of amino acid and polyamine metabolism in the small intestine. Amino Acids. 37, 123-129.
- Bird, J.G., Guthrie, A.S., Flynn, N.E. (2009). Acrylamide production in french fries may be reduced by cooking with olive oil. Journal of Undergraduate
- Haynes, T.E., Li, P., Li, X., Shimotori, K., Sato, H., Flynn, N.E., Wang, J., Knabe, D.A., and Wu, G. (2009). L-Glutamine or L-alanyl-glutamine prevents oxidant or endotoxin- induced death of neonatal enterocytes. Amino Acids. doi: 10.1007/s00726-009-0243-x.
- Bird, J.G., Guthrie, A.S. and Flynn, N.E. (2008). Recent advances on acrylamide chemistry and biology. Recent Advances in Nutrition. 2, 13-29.
